

COVID-19 Vaccines: How Did We Get Them So Fast
Credit: Cody Shipman, CoVPN
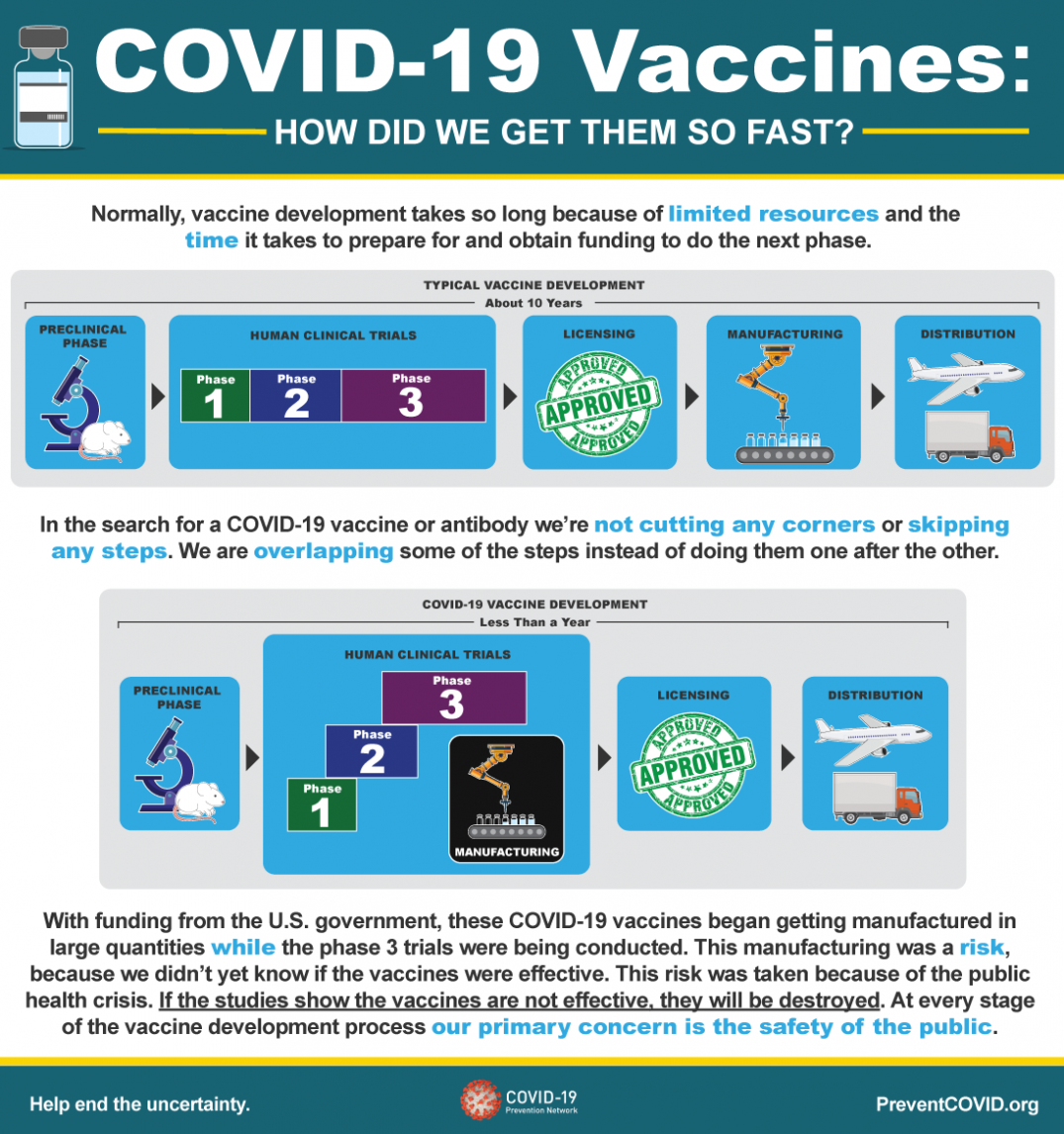
Normally, vaccine development takes so long because of limited resources and the time it takes to prepare for and obtain funding to do the next phase.
Typical Vaccine Development - About 10 Years
- Preclinical Phase
-
Human Clinical Trials
- Phase 1
- Phase 2
- Phase 3
- Licensing
- Manufacturing
- Distribution
In the search for a COVID-19 vaccine or antibody we’re not cutting any corners or skipping any steps. We are overlapping some of the steps instead of doing them one after the other.
COVID-19 Vaccine Development - Less Than a Year
- Preclinical Phase
-
Human Clinical Trials
- Phase 1
- Phase 2
- Phase 3/Manufacturing
- Licensing
- Distribution
With funding from the U.S. government, these COVID-19 vaccines began getting manufactured in large quantities while the phase 3 trials were being conducted. This manufacturing was a risk, because we didn’t yet know if the vaccines were effective. This risk was taken because of the public health crisis. If the studies show the vaccines are not effective, they will be destroyed. At every stage of the vaccine development process our primary concern is the safety of the public.
Content last reviewed on May 12, 2021
